Home Using the cellular handshake against the virus
Using the cellular handshake against the virus
The study used a genetically modified soluble form of ACE2 that mimics the protein found on human cell membranes.
Using the cellular handshake against the virus
In early November an interesting study was published in The Lancet Respiratory Medicine, describing the treatment of a severe COVID-19 patient with human recombinant soluble angiotensin-converting enzyme-2. By using the treatment, it was possible to see the disappearance of the virus swiftly from the patient's serum, nose, and lungs, as well as a reduction in the patients’ inflammatory markers. How was this achieved? And why is this so intriguing.
When we look at treating viruses, there are several strategies at work. You can prevent its spread by the use of social distancing or masks, you can use medications that prevent its replication (such as Tamiflu). Or you can stop the virus from entering the cells, the first step in viral replication.
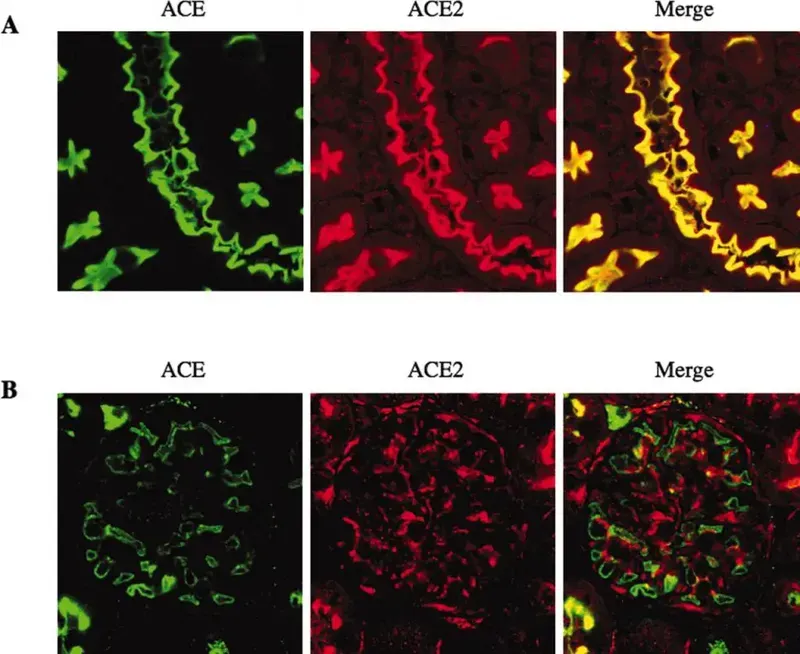
SARS-CoV-2 appears to enter human cells through the ACE2 receptor. ACE2 is a protein in the cell membrane found across the human body, especially the lungs, heart, kidney and small intestine. It function is related to the Renin-Angiotensin System, a mechanism by which the kidneys control your blood pressure.
Angiotensin-Converting Enzyme converts angiotensin 1 to angiotensin 2, a hormone that causes blood vessels to constrict. ACE 2 instead converts it to a protein that causes dilation of blood vessels. This likely protects these critical organs and ensure they receive sufficient blood to perform their tasks. ACE2 is being investigated as a possible target for blood pressure medications.
The study used a genetically modified soluble form of ACE2 that mimics the protein found on human cell membranes. By increasing its availability in the blood, the SARS-CoV-2 virus binds to this protein, resulting in its inactivation, rather than entering lung cells and causing lung injury. Lab studies have shown that it can reduce viral growth by as much as 1000 to 5000 times, and phase 1 studies show it to be safe to use in people.
The actual described case was in a 45-year-old woman, hospitalized with COVID, suffering from severe shortness of breath, cough and fatigue. She was first treated with hydroxychloroquine and anticoagulants, but this proved ineffective, and the virus was causing increasing damage to her lungs. Nine days after her symptoms started, she received the ACE2 treatment for a week, with no side effects. This resulted in an increase in ACE2 activity (seen from her blood results), but also a marked decrease in multiple inflammatory markers associated with COVID-19, as well as a sharp decrease in COVID 19 numbers. Indeed, the viral load dropped from 32,000 per mL to just 270 per mL two days after treatment started. She was also able to produce antibodies against the virus, so this did not impair her from getting immunity.
It is certainly a very interesting case, though questions remain on how feasible it would be (as well as how expensive) to manufacture large quantities of this protein receptor for treatment use, as well as how it fairs in clinical trials.